Written by Alison Derry, Université du Québec à Montréal (UQAM), Miguel Cañedo-Argüelles, Universitat de Barcelona & Stephanie J Melles, Toronto Metropolitan University. Photo credit: Author provided. Originally published in The Conversation.
Roads require de-icing strategies in northern regions, but this practice has negative effects on aquatic biodiversity.
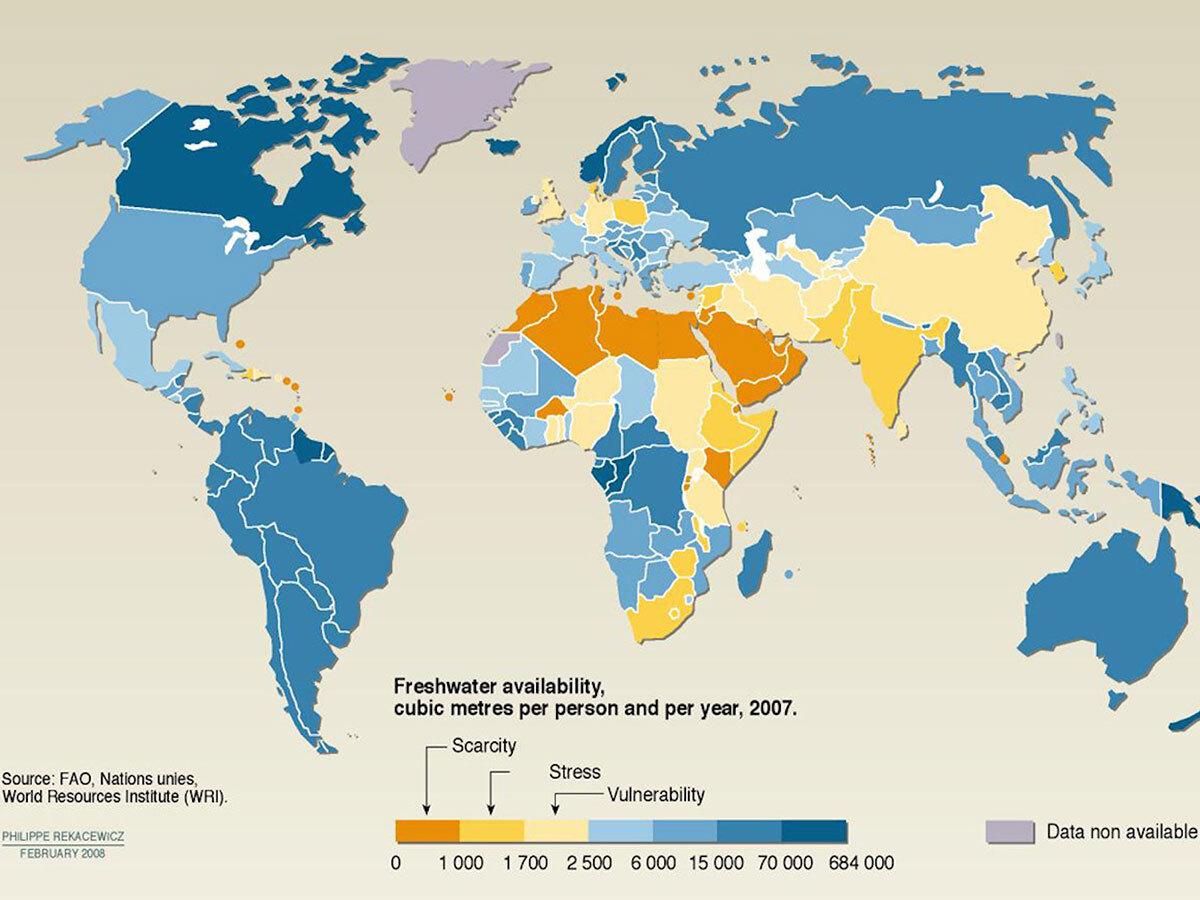
Freshwater ecosystems around the world are becoming saltier and saltier. Many human-driven factors contribute to freshwater salinization, including: irrigation, oil extraction, potash mining, and road de-icing.
As a result, salts enter waterways. But as bad news never comes alone, the salts are often accompanied by a toxic cocktail of other pollutants, whose combined toxicological effects are largely unknown.
Although the problem of rising freshwater salinization went largely unaddressed for many decades, it has gained considerable attention during the last 20 years.
Scientists around the world are working together to understand the ecological impacts of increasing salinization on aquatic biodiversity and food webs. Our ultimate goal? To examine the adequacy of water quality toxicity thresholds for the protection of aquatic life.
Salinization, a major problem
Canada is home to a majority of the world’s freshwater resources, mostly concentrated in the provinces of Ontario and Québec, where close to 5 million tons of road salt are applied annually to de-ice roads.
Combined with climate change and increasing frequency and duration of drought in many regions of the world, the problem is getting worse. This is a major concern. Why? Because the availability of freshwater resources will become a critical factor for humanity over the next 50 years.
Researchers from around the world mobilized
We recently presented a series of articles in a special issue on freshwater salinization in the journal Limnology and Oceanography Letters, published last February.
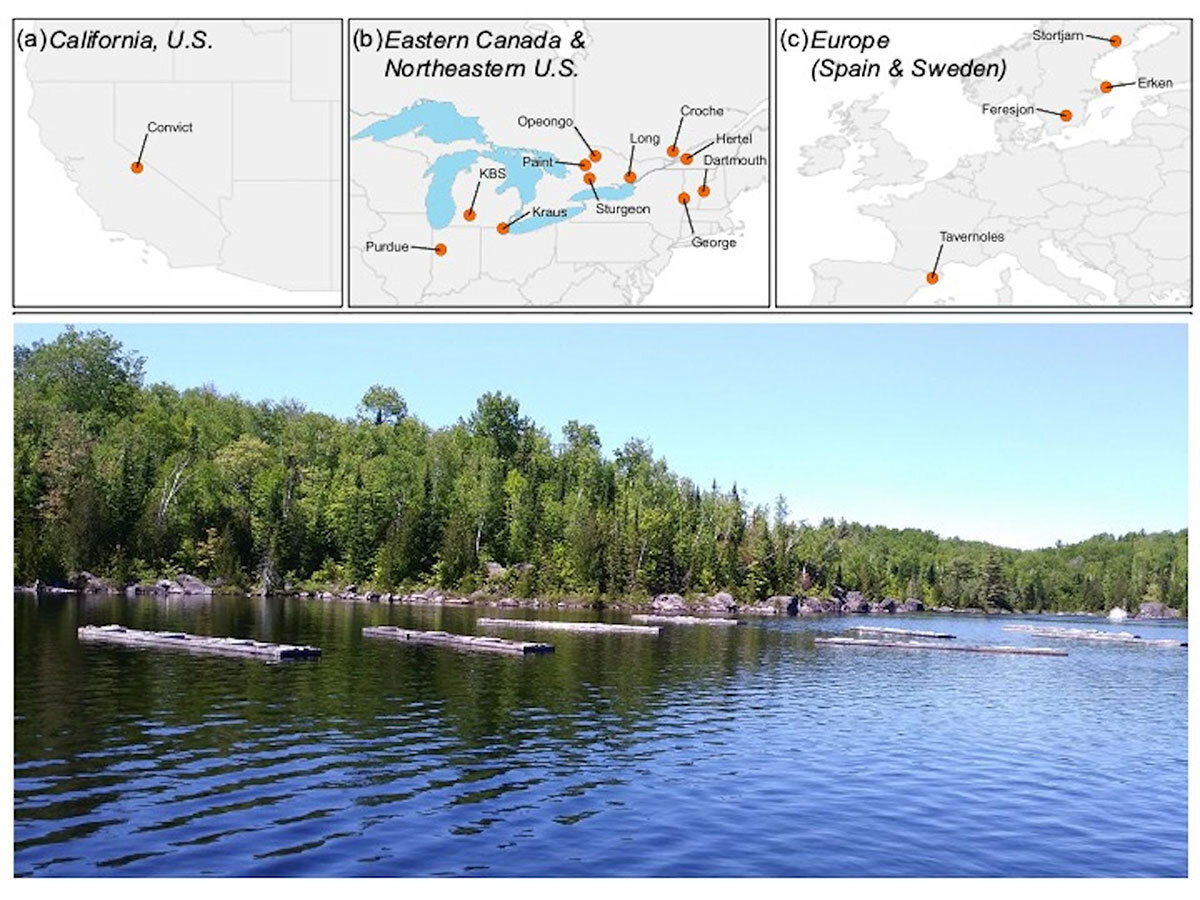
In this special issue, we focus on sodium chloride (NaCl), the same molecule found in table salt, as a key agent of freshwater salinization. We highlight a series of co-ordinated field experiments, conducted by researchers in North America and Europe, that have addressed the impacts of freshwater salinization on zooplankton (microscopic crustaceans) at a regional scale.
Zooplankton are an ecologically critical group in aquatic food webs and are often used as indicators to detect environmental change due to their sensitive ecological tolerances.
The main conclusions of these experiments are as follows:
- Water quality guidelines in Canada and the United States (standards) do not adequately protect freshwater zooplankton, which could lead to an increase in the abundance of algae, which the zooplankton feed on. This is because when zooplankton abundance decreases, especially for large grazers such as Daphnia, phytoplankton can proliferate under conditions of reduced predation;
- Salinization of freshwater systematically leads to a loss of abundance and diversity of zooplankton in all regions; and
- Individuals of the same zooplankton species do not all exhibit the same tolerance to salinity. Thus, this variation may interfere with our ability to predict community-level responses. Water quality guidelines may therefore need to be adjusted to become more region-specific.
A matter of regulation
Many questions remain unanswered. However, what we do now know is that long-term water quality guidelines (Canada: 120 mg Cl⁻¹L⁻¹; United States: 230 mg Cl⁻¹L⁻¹) and in the short term (Canada: 640 mg Cl⁻¹L⁻¹; United States: 860 mg Cl⁻¹L⁻¹) for chloride concentrations are too high to protect aquatic life in Canada and in the United States. For reference, a pinch of salt in a pot of water corresponds to approximately 0.3 g of Cl⁻¹/L⁻¹. In other words, adverse effects are observed at much lower concentrations. Regulations in Canada and the United States should therefore be reviewed. In Europe, the water quality standards for salinity for the protection of aquatic life in freshwater ecosystems are mostly absent.
The importance of taking concrete action
Water quality guidelines for the protection of aquatic life are generally established using laboratory tests (called toxicological tests) on a single species.
However, aquatic habitats harbour a complex array of predators, prey, competitors, and pathogens, the interactions of which can limit our ability to predict the responses of communities and species to pollutants .
Thus, the collective research published in this special issue also highlights the importance of understanding ecological responses in multi-species communities in natural settings to assess the responses of freshwater life to human impacts.

Overall, we should develop alternative applications and technologies that are more sustainable and efficient.
We also need to establish more appropriate water quality guidelines to improve controls on salts entering our freshwater environments to reduce adverse effects on aquatic life and the quality of our freshwater resources.

